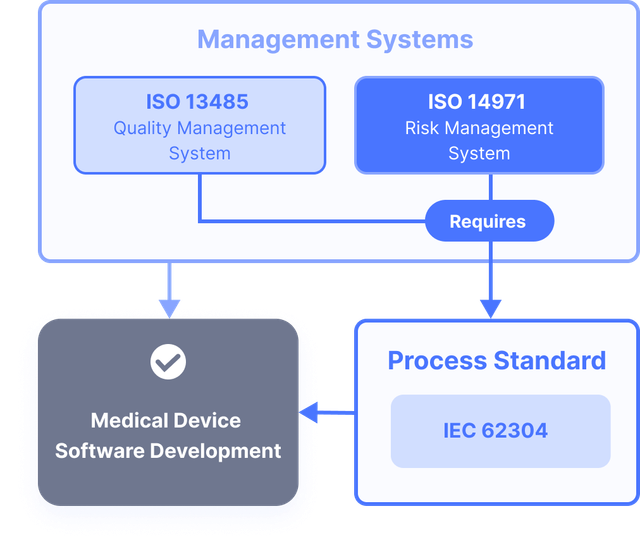
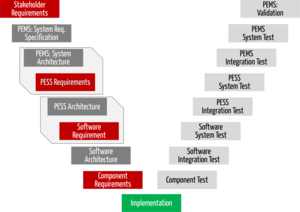
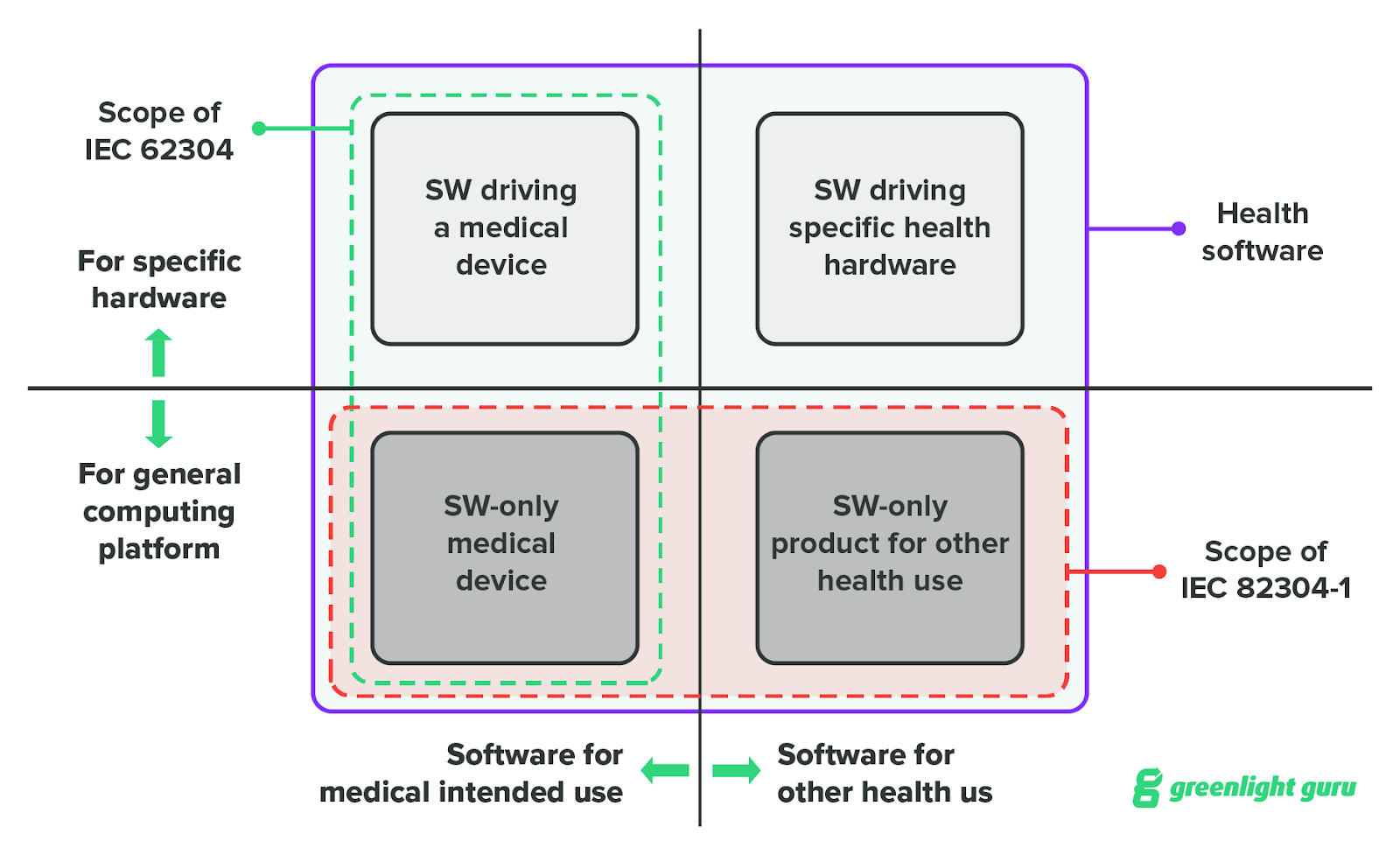
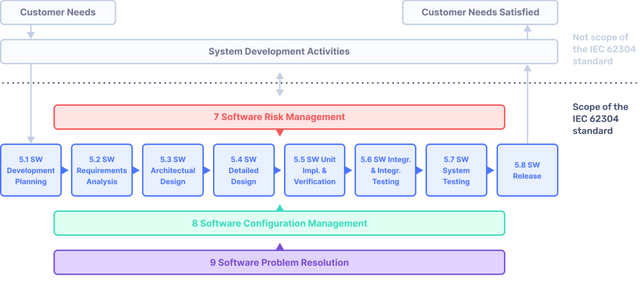
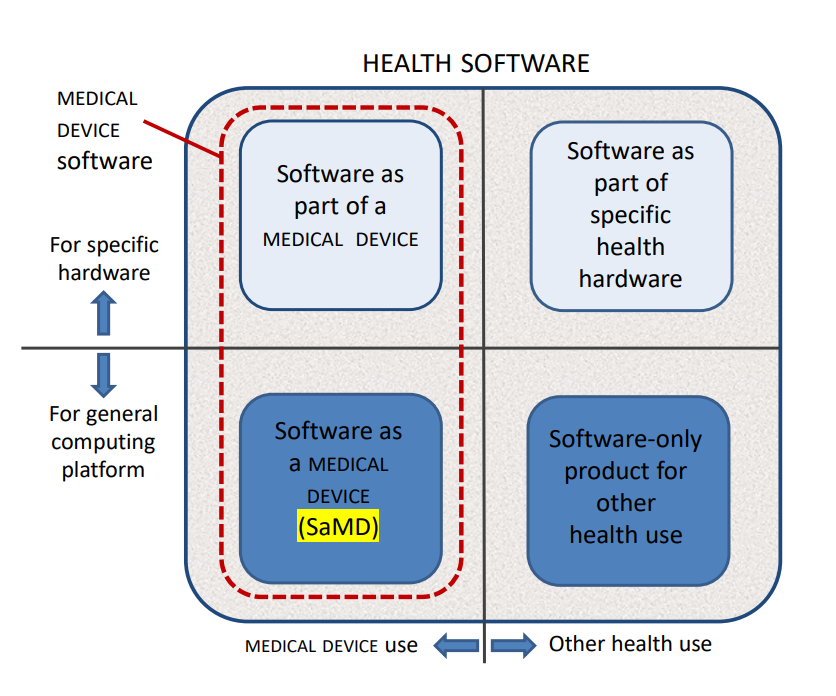
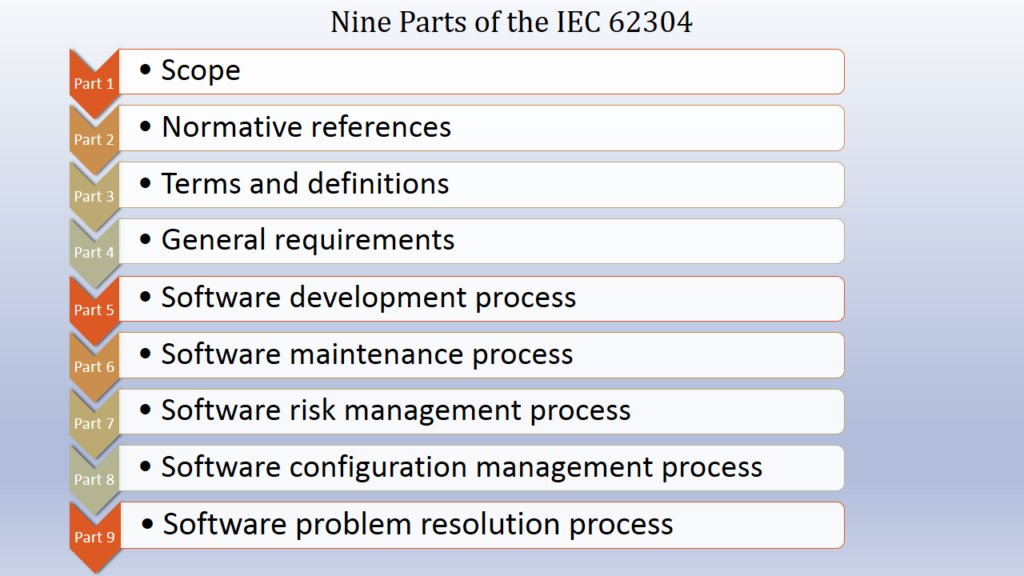
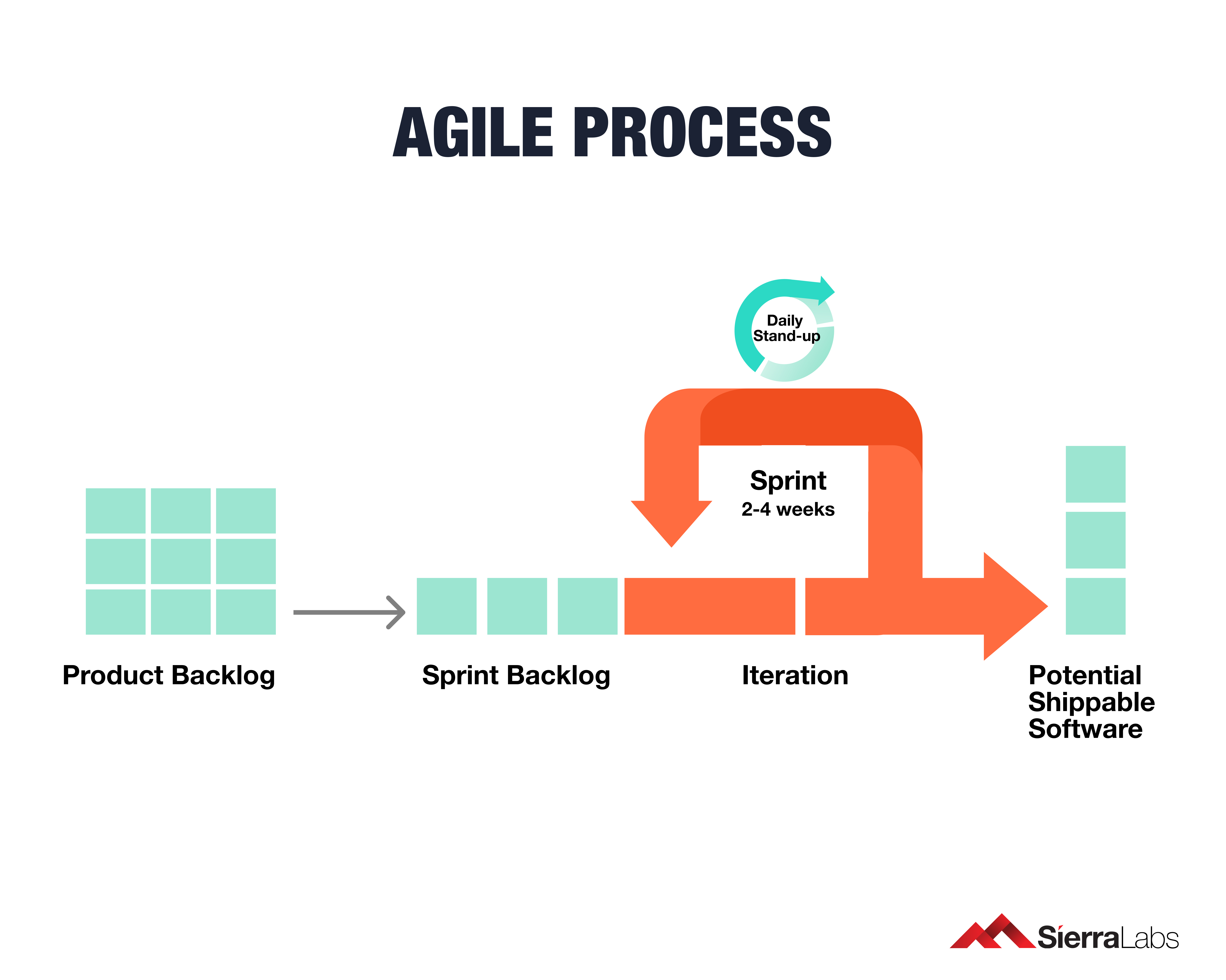
Implementing IEC 62304 for Safe and Effective Medical Device Software, PART 2 - Medical Design Briefs

Implementing IEC 62304 for Safe and Effective Medical Device Software — PART 1 - Medical Design Briefs

IEC 62304: Medical Device Software LifeCycle Processes | by Dr Stephen Odaibo | The Blog of RETINA-AI Health, Inc. | Medium
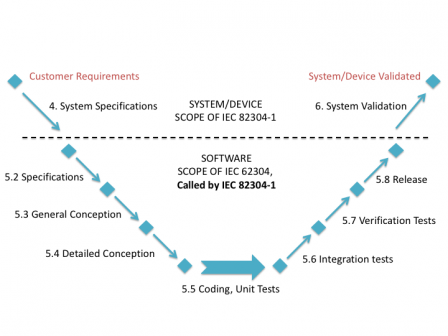
IEC 82304-1 - Consequences on agile software development processes - Software in Medical Devices, by MD101 Consulting

Relationship of EN 62304 to other standards. Compliance with the EN... | Download Scientific Diagram